Prof. Ruth Ashery-Padan
Principal investigator
Prof. Ruth Ashery-Padan
Principal investigator
Prof. Ruth Ashery-Padan
Principal investigator
Prof. Ruth Ashery-Padan
Principal investigator
Prof. Ruth Ashery-Padan
Principal investigator
Research
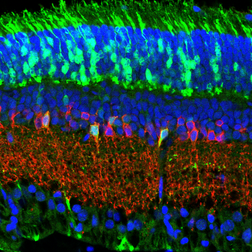
Organogenesis of the eye takes place through a series of interactions between transient embryonic structures that undergo morphogenetic events. Transcription regulators and signaling pathways orchestrate these events by regulating cell cycle, cell survival, morphogenesis, specification and differentiation to various ocular organs. Our studies focus on understanding the molecular mechanisms that control the development of the visual system in mammals. We establish and employ transgenic mouse lines as well as in-vivo electroporation for state-of-the-art functional studies of genes in vivo. This is combined with analyses of gene expression profiles, biochemical and cell culture studies of transcription factor activity on target genes. Studies in ocular cells types generated from human stem-cells are employed in the lab to uncover the molecular mechanism of differentiation of human lineages and relevance to human diseases. Our findings lead to the discovery of the gene networks controlling organogenesis of the eye in mammals.
Pax6 is one of the transcriptional regulators that has been extensively investigated in the lab. This gene is essential and sufficient for eye formation in vertebrate and invertebrate species and is pivotal for development of the CNS, olfactory system and pancreas. Our findings have revealed Pax6-dependent gene networks in developing lens retina, iris and retinal pigmented epithelium, implicating interactions with chromatin modifiers and demonstrating the mechanism by which a single developmental regulator acquires tissue-specific activities (1-20).
Our group further investigates roles of key factors for regulating the coupling between cell-cycle exit and onset of differentiation in the developing nervous system including Notch1 (21), Zeb2 (22,23) and the Ldb-Lhx transcriptional complex (24) in the retinal progenitors and how each of these factors impact the eventual cell numbers and cell-types generated in the retina.
Another topic of research in the lab is on the roles of microRNAs (7, 25, 26, 27) and their contribution to the coordinate development of neuronal and non-neuronal ocular structures.
These and ongoing studies in the lab contribute to understanding the etiology of complex diseases, toward a better prediction of individual susceptibility and the design of stem-cell based models and future therapies for these diseases.
-
Cohen-Tayar, Y., Cohen, H., Mitiagin, Y., Abarbanel, Z., Levy, C., Idelson, M. et al. Pax6 regulation of Sox9 in the retinal pigmented epithelium controls its timely differentiation and choroid vasculature development. Development, (2018).
http://www.ncbi.nlm.nih.gov/pubmed/29986868
-
Remez, L. A., Onishi, A., Menuchin-Lasowski, Y., Biran, A., Blackshaw, S., Wahlin, K. J. et al. Pax6 is essential for the generation of late-born retinal neurons and for inhibition of photoreceptor-fate during late stages of retinogenesis. Dev Biol, (2017). http://www.ncbi.nlm.nih.gov/pubmed/28993200
-
Sun, J., Rockowitz, S., Xie, Q., Ashery-Padan, R., Zheng, D. & Cvekl, A. Identification of in vivo DNA-binding mechanisms of Pax6 and reconstruction of Pax6-dependent gene regulatory networks during forebrain and lens development. Nucleic Acids Res, (2015). http://www.ncbi.nlm.nih.gov/pubmed/26138486
-
Sun, J., Zhao, Y., McGreal, R., Cohen-Tayar, Y., Rockowitz, S., Wilczek, C. et al. Pax6 associates with H3K4-specific histone methyltransferases Mll1, Mll2, and Set1a and regulates H3K4 methylation at promoters and enhancers. Epigenetics Chromatin 9, 37, (2016). http://www.ncbi.nlm.nih.gov/pubmed/27617035
-
Raviv, S., Bharti, K., Rencus-Lazar, S., Cohen-Tayar, Y., Schyr, R., Evantal, N. et al. PAX6 regulates melanogenesis in the retinal pigmented epithelium through feed-forward regulatory interactions with MITF. PLoS Genet 10, e1004360, (2014). http://www.ncbi.nlm.nih.gov/pubmed/24875170
-
Wolf, L., Harrison, W., Huang, J., Xie, Q., Xiao, N., Sun, J. et al. Histone posttranslational modifications and cell fate determination: lens induction requires the lysine acetyltransferases CBP and p300. Nucleic Acids Res, (2013)
http://www.ncbi.nlm.nih.gov/pubmed/24038357
-
Shaham, O., Gueta, K., Mor, E., Oren-Giladi, P., Grinberg, D., Xie, Q. et al. Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204. PLoS Genet 9, e1003357, (2013)
http://www.ncbi.nlm.nih.gov/pubmed/23516376
-
Farhy, C., Elgart, M., Shapira, Z., Oron-Karni, V., Yaron, O., Menuchin, Y. et al. Pax6 is required for normal cell-cycle exit and the differentiation kinetics of retinal progenitor cells. PLoS One 8, e76489, (2013)
http://www.ncbi.nlm.nih.gov/pubmed/24073291
-
Huang, J., Rajagopal, R., Liu, Y., Dattilo, L. K., Shaham, O., Ashery-Padan, R. et al. The mechanism of lens placode formation: a case of matrix-mediated morphogenesis. Dev Biol 355, 32-42, (2011)
http://www.ncbi.nlm.nih.gov/pubmed/21540023
-
Kroeber, M., Davis, N., Holzmann, S., Kritzenberger, M., Shelah-Goraly, M., Ofri, R. et al. Reduced expression of Pax6 in lens and cornea of mutant mice leads to failure of chamber angle development and juvenile glaucoma. Hum Mol Genet, (2010)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20538882
-
He, S., Limi, S., McGreal, R. S., Xie, Q., Brennan, L. A., Kantorow, W. L. et al. Chromatin remodeling enzyme Snf2h regulates embryonic lens differentiation and denucleation. Development 143, 1937-1947, (2016)
http://www.ncbi.nlm.nih.gov/pubmed/27246713
-
Tuoc, T. C., Radyushkin, K., Tonchev, A. B., Pinon, M. C., Ashery-Padan, R., Molnar, Z. et al. Selective cortical layering abnormalities and behavioral deficits in cortex-specific Pax6 knock-out mice. J Neurosci 29, 8335-8349, (2009)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19571125
-
Smith, A. N., Miller, L. A., Radice, G., Ashery-Padan, R. & Lang, R. A. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development 136, 2977-2985, (2009)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19666824
-
Shaham, O., Smith, A. N., Robinson, M. L., Taketo, M. M., Lang, R. A. & Ashery-Padan, R. Pax6 is essential for lens fiber cell differentiation. Development 136, 2567-2578, (2009)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19570848
-
Davis, N., Yoffe, C., Raviv, S., Antes, R., Berger, J., Holzmann, S. et al. Pax6 dosage requirements in iris and ciliary body differentiation. Dev Biol 333, 132-142, (2009)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19563798
-
Oron-Karni, V., Farhy, C., Elgart, M., Marquardt, T., Remizova, L., Yaron, O. et al. Dual requirement for Pax6 in retinal progenitor cells. Development 135, 4037-4047, (2008)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19004853
-
Davis-Silberman, N., Kalich, T., Oron-Karni, V., Marquardt, T., Kroeber, M., Tamm, E. R. et al. Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum Mol Genet 14, 2265-2276, (2005)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15987699
-
Baumer, N., Marquardt, T., Stoykova, A., Ashery-Padan, R., Chowdhury, K. & Gruss, P. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129, 4535-4545, (2002)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12223410
-
Marquardt, T., Ashery-Padan, R., Andrejewski, N., Scardigli, R., Guillemot, F. & Gruss, P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43-55, (2001)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11301001
-
Ashery-Padan, R., Marquardt, T., Zhou, X. & Gruss, P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev 14, 2701-2711, (2000)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11069887
-
Yaron, O., Farhy, C., Marquardt, T., Applebury, M. & Ashery-Padan, R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development 133, 1367-1378, (2006)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16510501
-
Menuchin-Lasowski, Y., Dagan, B., Conidi, A., Cohen-Gulkar, M., David, A., Ehrlich, M. et al. Zeb2 regulates the balance between retinal interneurons and muller glia by inhibition of BMP-Smad signaling. Dev Biol, (2020)
http://www.ncbi.nlm.nih.gov/pubmed/32950463
-
Menuchin-Lasowski, Y., Oren-Giladi, P., Xie, Q., Ezra-Elia, R., Ofri, R., Peled-Hajaj, S. et al. Sip1 regulates the generation of the inner nuclear layer retinal cell lineages in mammals. Development 143, 2829-2841, (2016)
http://www.ncbi.nlm.nih.gov/pubmed/27385012
-
Gueta, K., David, A., Cohen, T., Menuchin-Lasowski, Y., Nobel, H., Narkis, G. et al. The stage-dependent roles of Ldb1 and functional redundancy with Ldb2 in mammalian retinogenesis. Development, (2016)
http://www.ncbi.nlm.nih.gov/pubmed/27697904 -
Davis, N., Mor, E. & Ashery-Padan, R. Roles for Dicer1 in the patterning and differentiation of the optic cup neuroepithelium. Development 138, 127-138, (2011)
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21138975
-
Ohana, R., Weiman-Kelman, B., Raviv, S., Tamm, E. R., Pasmanik-Chor, M., Rinon, A. et al. MicroRNAs are essential for differentiation of the retinal pigmented epithelium and maturation of adjacent photoreceptors. Development 142, 2487-2498, (2015)
http://www.ncbi.nlm.nih.gov/pubmed/26062936
-
Wolf, L., Gao, C. S., Gueta, K., Xie, Q., Chevallier, T., Podduturi, N. R. et al. Identification and Characterization of FGF2-Dependent mRNA:microRNA Networks During Lens Fiber Cell Differentiation. G3 (Bethesda), (2013)
http://www.ncbi.nlm.nih.gov/pubmed/24142921